
Current state of U.S. Food and Drug Administration regulation for cellular and gene therapy products: potential cures on the horizon - Cytotherapy
Comparison of FDA accelerated vs regular pathway approvals for lung cancer treatments between 2006 and 2018 | PLOS ONE

Drugs, Devices, and the FDA: Part 2: An Overview of Approval Processes: FDA Approval of Medical Devices - ScienceDirect

FDA efficiency for approval process of COVID-19 therapeutics | Infectious Agents and Cancer | Full Text
U.S. FDA Approval Summary: Nivolumab for Treatment of Unresectable or Metastatic Melanoma Following Progression on Ipilimumab

Drugs, Devices, and the FDA: Part 2: An Overview of Approval Processes: FDA Approval of Medical Devices - ScienceDirect

Fillable Online fda SUMMARY BASIS FOR APPROVAL PLA - Food and Drug ... - fda Fax Email Print - pdfFiller

Fillable Online fda Product Approval Information Summary Basis of Approval OCTAGAM 5% - fda Fax Email Print - pdfFiller
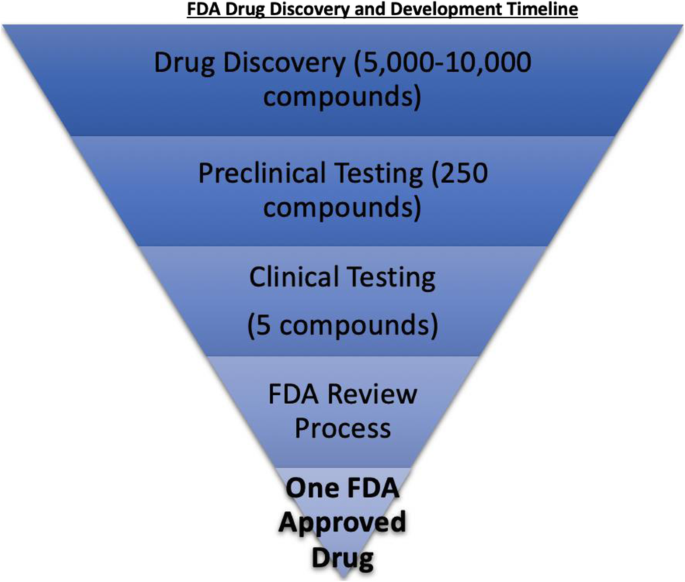
FDA efficiency for approval process of COVID-19 therapeutics | Infectious Agents and Cancer | Full Text

Introduction to EPARs and FDA Approval Packages: Finding and Analyzing Unpublished Information about Pivotal Studies 23 June 2008 Session Chair: Amy N. - ppt download
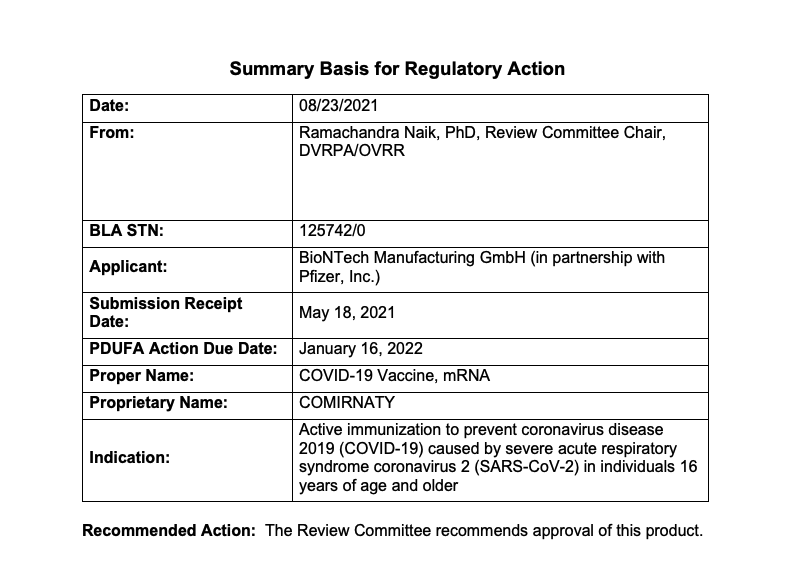
Alexander Tin on Twitter: "As promised yesterday, @US_FDA has posted its "Summary Basis for Regulatory Action" document on @pfizer-@BioNTech_Group's COVID-19 vaccines full approval https://t.co/gytKOptwYf https://t.co/TZrlSrhm2C" / Twitter










