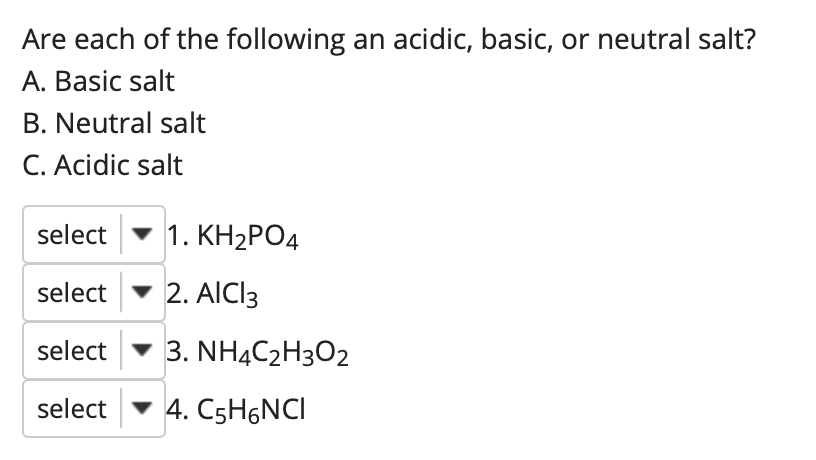
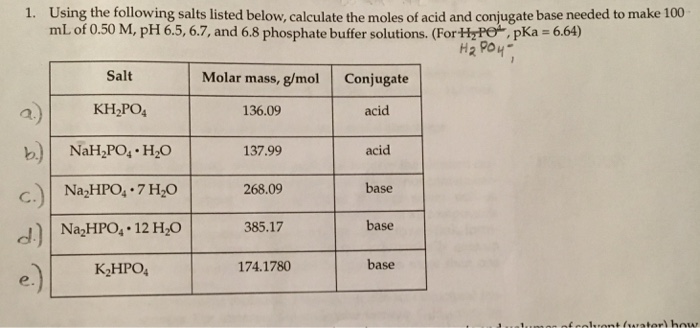
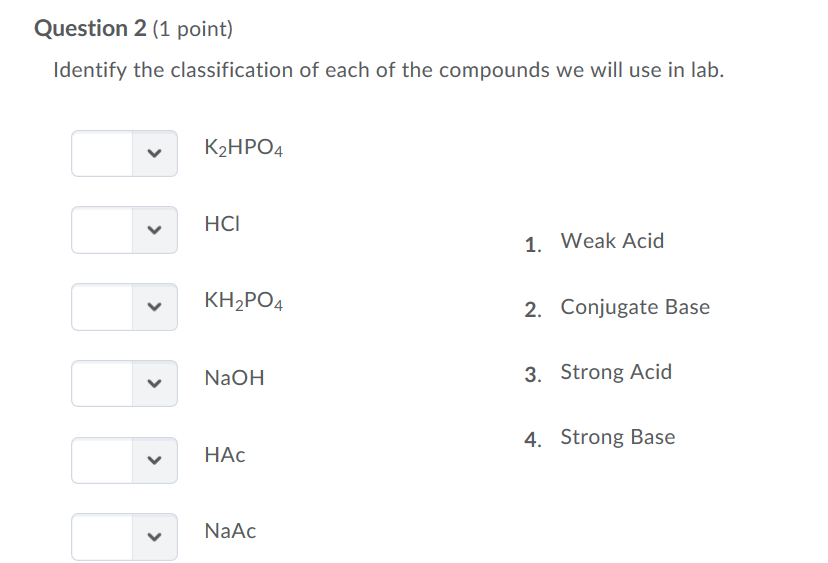
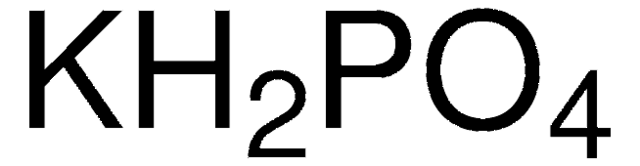![Potassium Phosphate Monobasic [KH2PO4] 99+% Fine Crystals 1.5 Lb in Three Space-Saver Bottles: Amazon.com: Industrial & Scientific Potassium Phosphate Monobasic [KH2PO4] 99+% Fine Crystals 1.5 Lb in Three Space-Saver Bottles: Amazon.com: Industrial & Scientific](https://m.media-amazon.com/images/I/515VzhubFGL._SR600%2C315_PIWhiteStrip%2CBottomLeft%2C0%2C35_PIStarRatingFOUR%2CBottomLeft%2C360%2C-6_SR600%2C315_ZA6%2C445%2C290%2C400%2C400%2CAmazonEmberBold%2C12%2C4%2C0%2C0%2C5_SCLZZZZZZZ_FMpng_BG255%2C255%2C255.jpg)
Potassium Phosphate Monobasic [KH2PO4] 99+% Fine Crystals 1.5 Lb in Three Space-Saver Bottles: Amazon.com: Industrial & Scientific

Phase Equilibrium for the Ternary System KH2PO4 + NaH2PO4 + H2O at 303.15 K | Journal of Chemical & Engineering Data
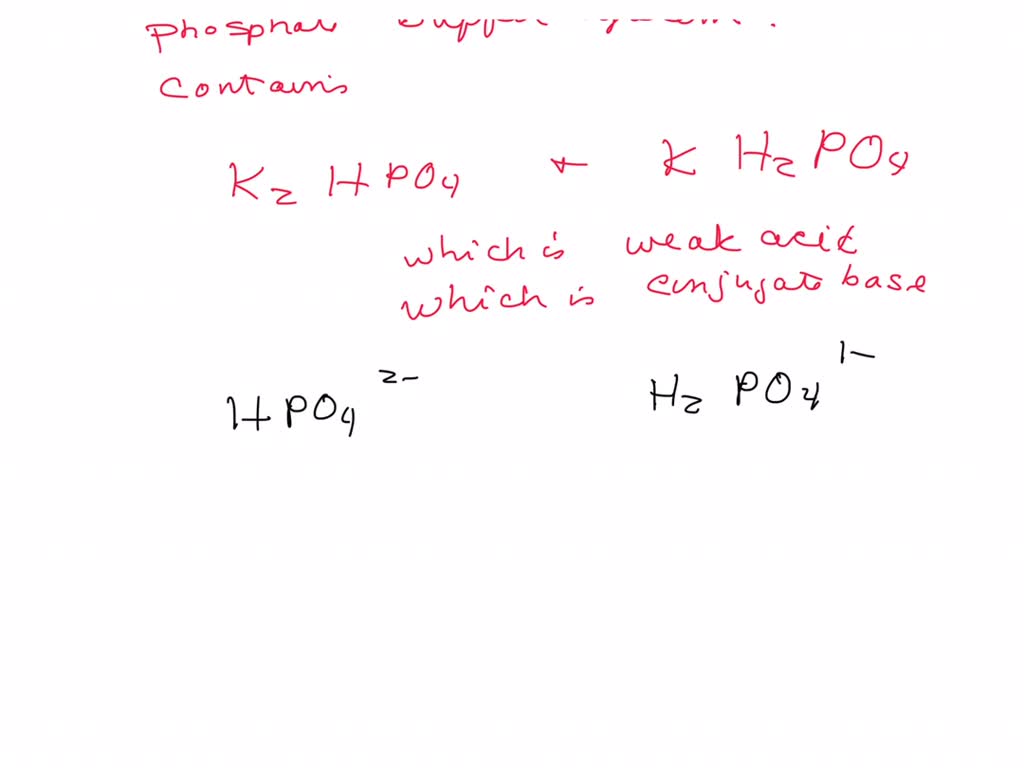
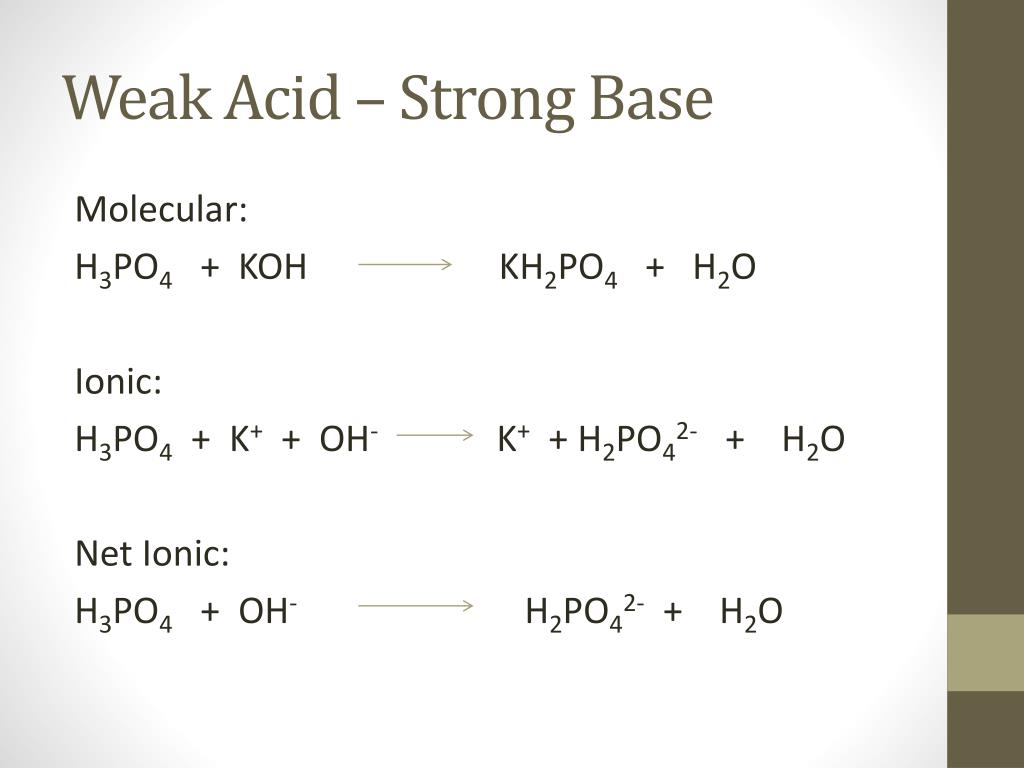
SOLVED: In the phosphate buffer system containing K2HPO4 and KH2PO4, what is the weak acid? What is its conjugate base?

Enhanced Second-Harmonic-Generation Response in a KH2PO4-Type Calcium Nitrate Carboxylate with Unusual Three-Dimensional Inorganic and Organic Connections | Inorganic Chemistry

Nitrogen loss reduction by adding KH2PO4-K2HPO4 buffer solution during composting of sewage sludge - ScienceDirect

Plants | Free Full-Text | Effects of Spraying KH2PO4 on Flag Leaf Physiological Characteristics and Grain Yield and Quality under Heat Stress during the Filling Period in Winter Wheat

Effect of KH2PO4 concentration in the mobile phase on the retention... | Download Scientific Diagram

14.87 | Calculate the pH of a buffer solution prepared from 0.155 mol of phosphoric acid, 0.250 mole - YouTube