MEP maps of isolated TrHX, TrH2X and H2Y molecules (Tr = Ga, In; X = F,... | Download Scientific Diagram
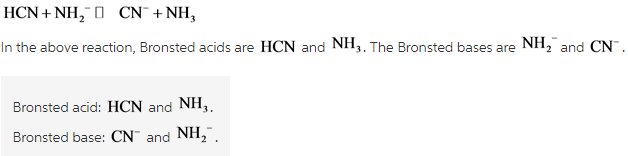
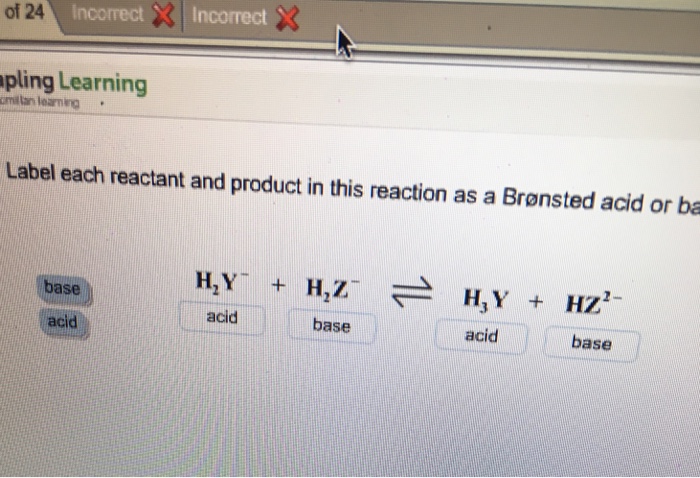
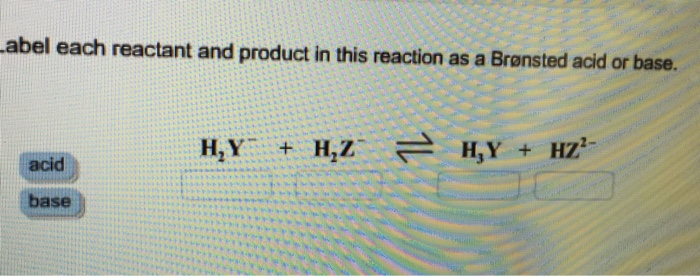
Label each reactant and product in this reaction as a Bronsted acid or base - Home Work Help - Learn CBSE Forum

Nonaqueous Chemistry of Group 4 Oxo Clusters and Colloidal Metal Oxide Nanocrystals | Chemical Reviews
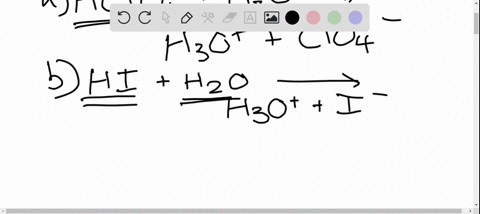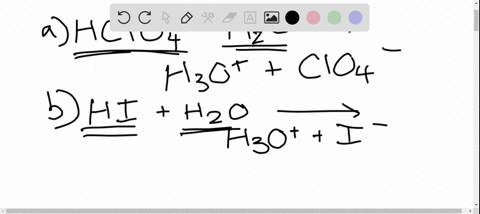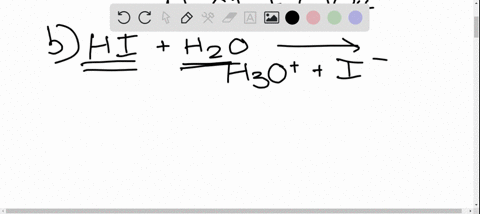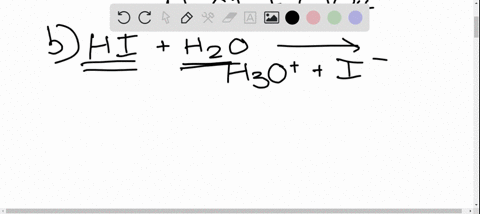
SOLVED: In the reaction between H2Y - and H2Z - , if H2Y - acts like a base, and H2Z - acts like an acid, the products of the reaction are: A)
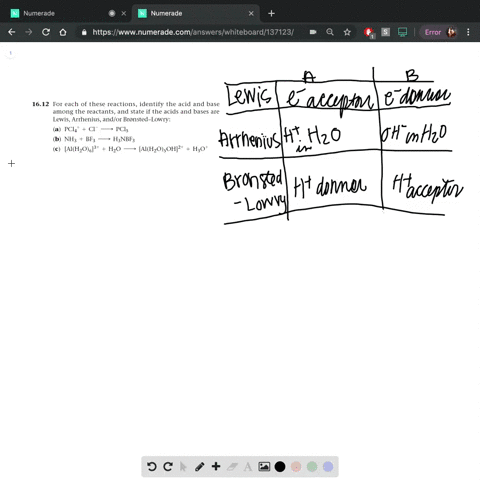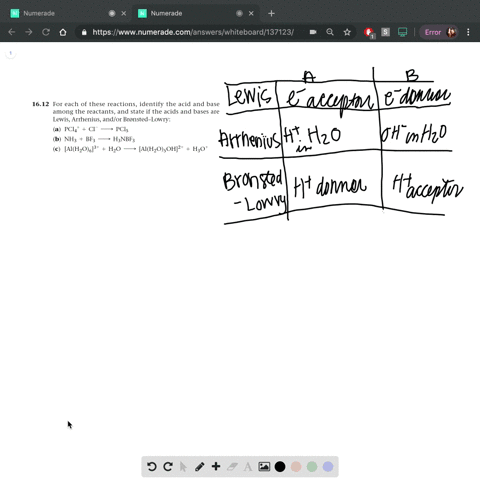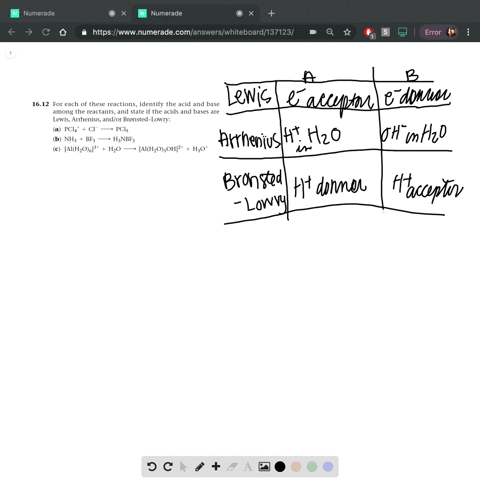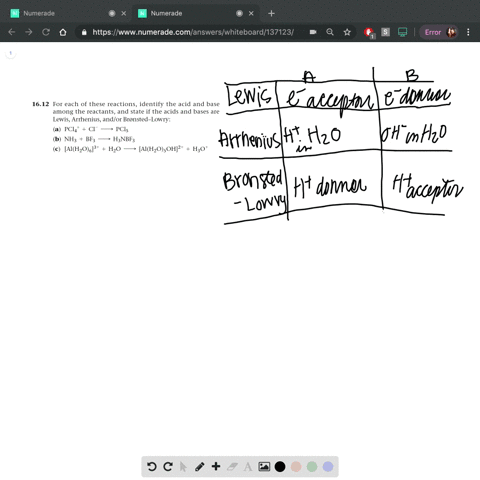
SOLVED: In the reaction between H2Y - and H2Z - , if H2Y - acts like a base, and H2Z - acts like an acid, the products of the reaction are: A)

Highly Selective Surface Lewis Acid−Base Reaction: Trimethylamine on Si(100)c(4×2) | The Journal of Physical Chemistry B

MEP maps of isolated TrHX, TrH2X and H2Y molecules (Tr = Ga, In; X = F,... | Download Scientific Diagram

SOLVED: 30.At what pH would you expect the aminoacid prolinePto be fully protonated?pK=1.952,pK2=10.640 A.1.952 B.1.750 C.2.500 D.10.640 E.7.000 31.Given pKvalues of citric acid2-hydroxypropane-1,2,3-tricarboxylic acid,pK=3.128,pK=4.761,and pK3= 6.396 ...

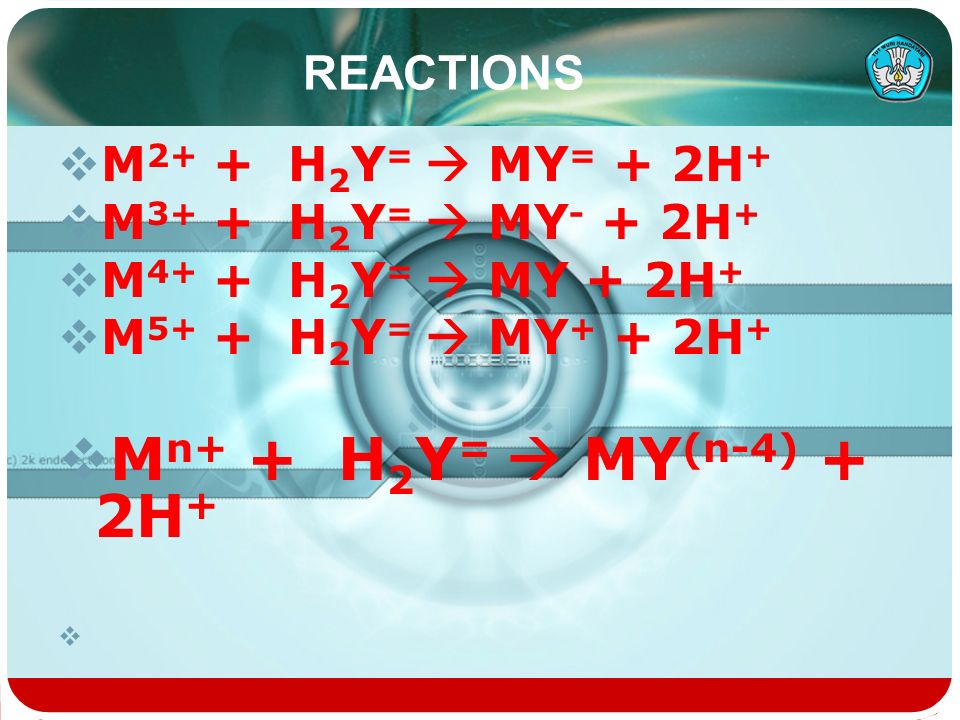
25.0cm<sup>3</sup> of 0.12M potassium hydroxide solution required 30.0cm<sup>3</sup> of a solution of a dibasic acid (H<sub>2</sub>Y) for complete neutralization. The acid contained 3.15g per 500cm<sup>3</sup>...

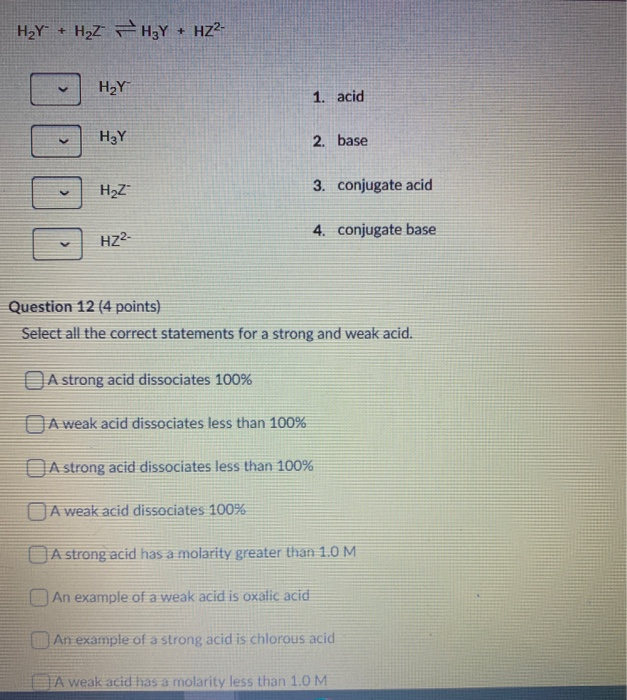
SOLVED: Identify each reactant and product in this reaction as a Bronsted acid or base. Hz Y- + HzZ acid base H; Y + HZ2 - base acid Answer Bank base acid
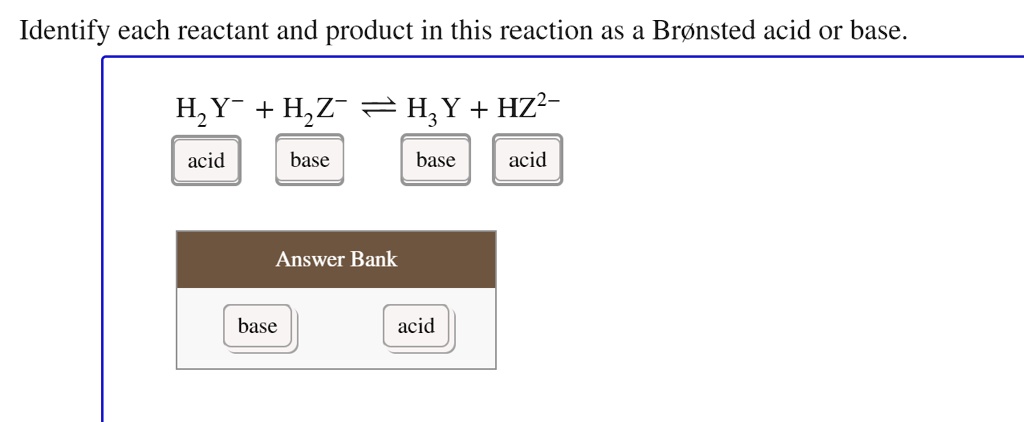
SOLVED: Identify each reactant and product in this reaction as a Bronsted acid or base. Hz Y- + HzZ acid base H; Y + HZ2 - base acid Answer Bank base acid
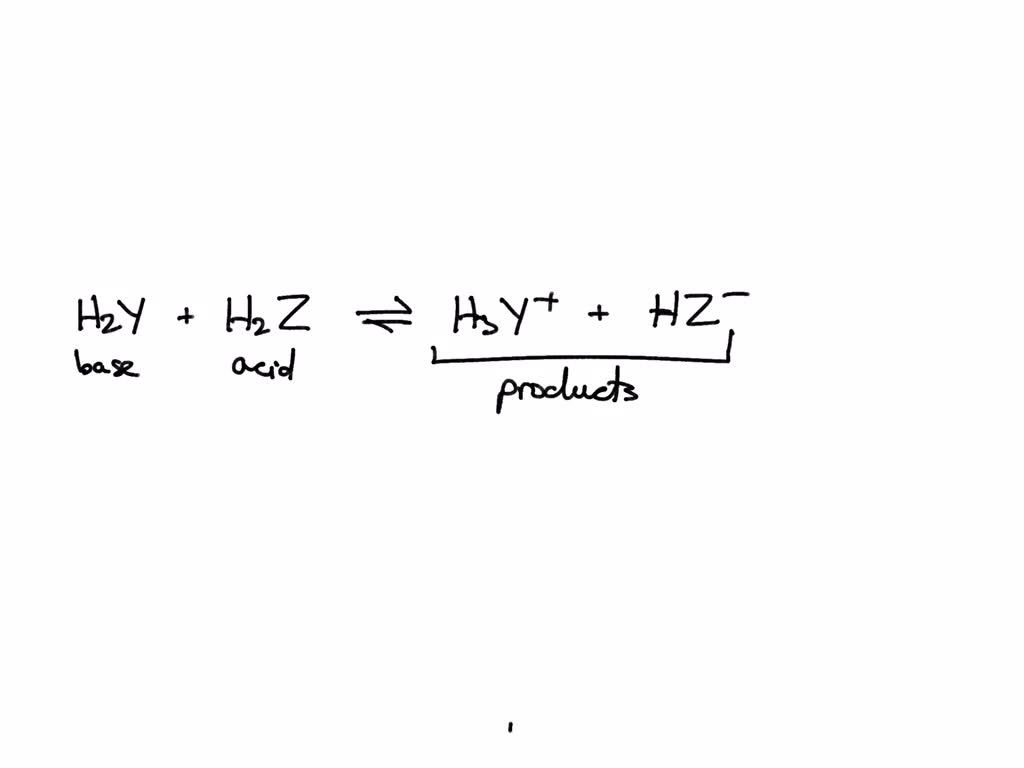
SOLVED: In the reaction between H2Y - and H2Z - , if H2Y - acts like a base, and H2Z - acts like an acid, the products of the reaction are: A)

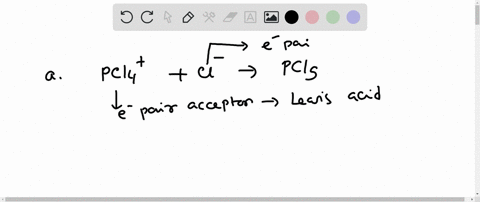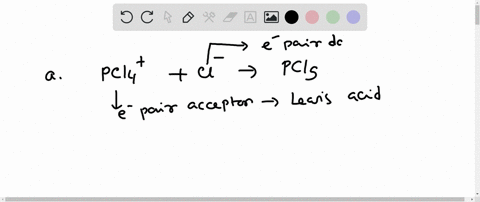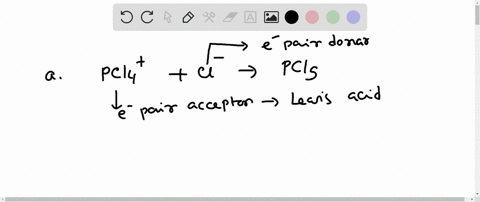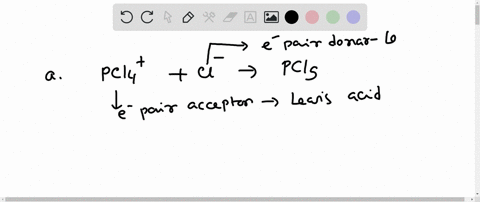
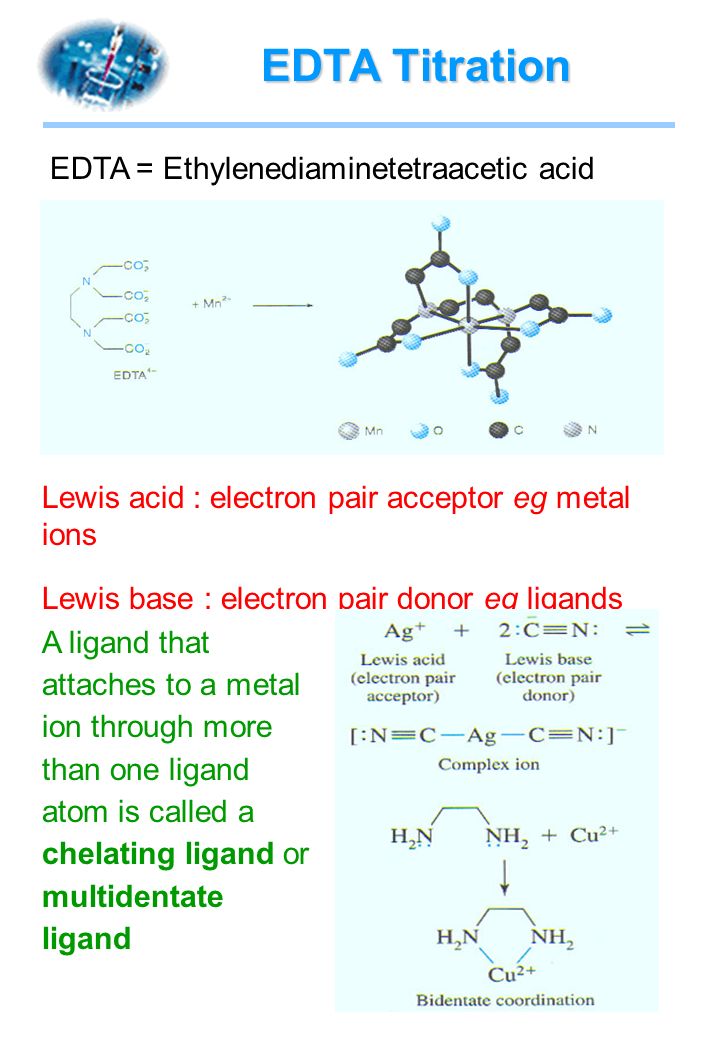
Complete the equation for the reaction between the following Lewis acid-base pair. Label which starting material is the Lewis acid and which is the Lewis base, and use curved arrows to show

LES 3 CHENES COLOR & SOIN Soin Lavant Cheveux Colorés Clairs (250 ml) : Amazon.ca: Health & Personal Care

The aqueous solution of transition metal salt changes colour from pink to blue, when concentrated hydrochloric acid is added to it. The change in colour is due to

Complete the equation for the reaction between the following Lewis acid-base pair. Label which starting material is the Lewis acid and which is the Lewis base, and use curved arrows to show