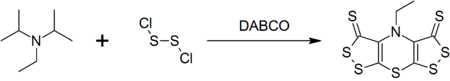![Selective Syntheses of Bis[1,2]dithiolo[1,4]thiazines and Bis[1,2]dithiolopyrroles from Hünig's Base | The Journal of Organic Chemistry Selective Syntheses of Bis[1,2]dithiolo[1,4]thiazines and Bis[1,2]dithiolopyrroles from Hünig's Base | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/jo971864e/asset/images/large/jo971864en00001.jpeg)
Selective Syntheses of Bis[1,2]dithiolo[1,4]thiazines and Bis[1,2]dithiolopyrroles from Hünig's Base | The Journal of Organic Chemistry

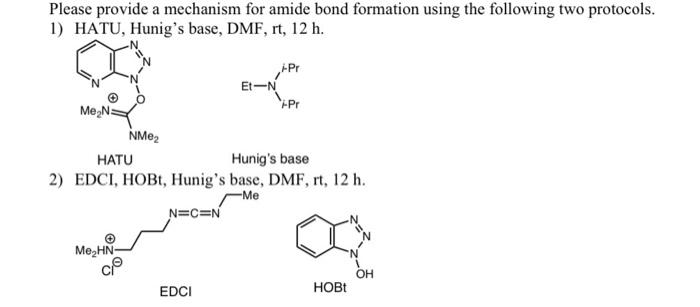
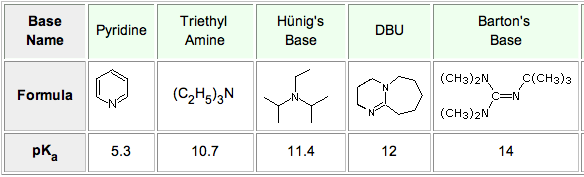
Ru-TsDPEN with Formic Acid/Hünig's Base for Asymmetric Transfer Hydrogenation, a Practical Synthesis of Optically Enriched N-Propyl Pantolactam | The Journal of Organic Chemistry
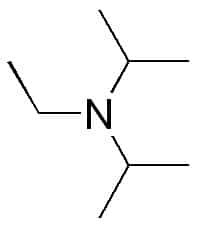
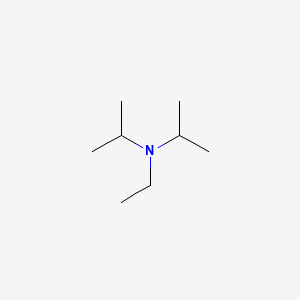
7087-68-5 | N,N-Diisopropylethylamine | 1,1'-Dimethyltriethylamine; Bis(1-methylethyl)ethylamine; DIEA; DIPEA; Diisopropylethylamine; Ethyl-N,N-diisopropylamine; Ethyldiisopropylamine; Huenig's base; Hunig's base; Hunig's reagent; N,N-Bis(1-methylethyl ...
![PDF] A SIMPLE AND HIGHLY EFFICIENT SYNTHESIS OF QUINOLINE TERTIARY AMINES CATALYZED BY HUNIG'S BASE | Semantic Scholar PDF] A SIMPLE AND HIGHLY EFFICIENT SYNTHESIS OF QUINOLINE TERTIARY AMINES CATALYZED BY HUNIG'S BASE | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/3c974f939a32262aeed197ab08f1ab631c012982/4-Table2-1.png)
PDF] A SIMPLE AND HIGHLY EFFICIENT SYNTHESIS OF QUINOLINE TERTIARY AMINES CATALYZED BY HUNIG'S BASE | Semantic Scholar

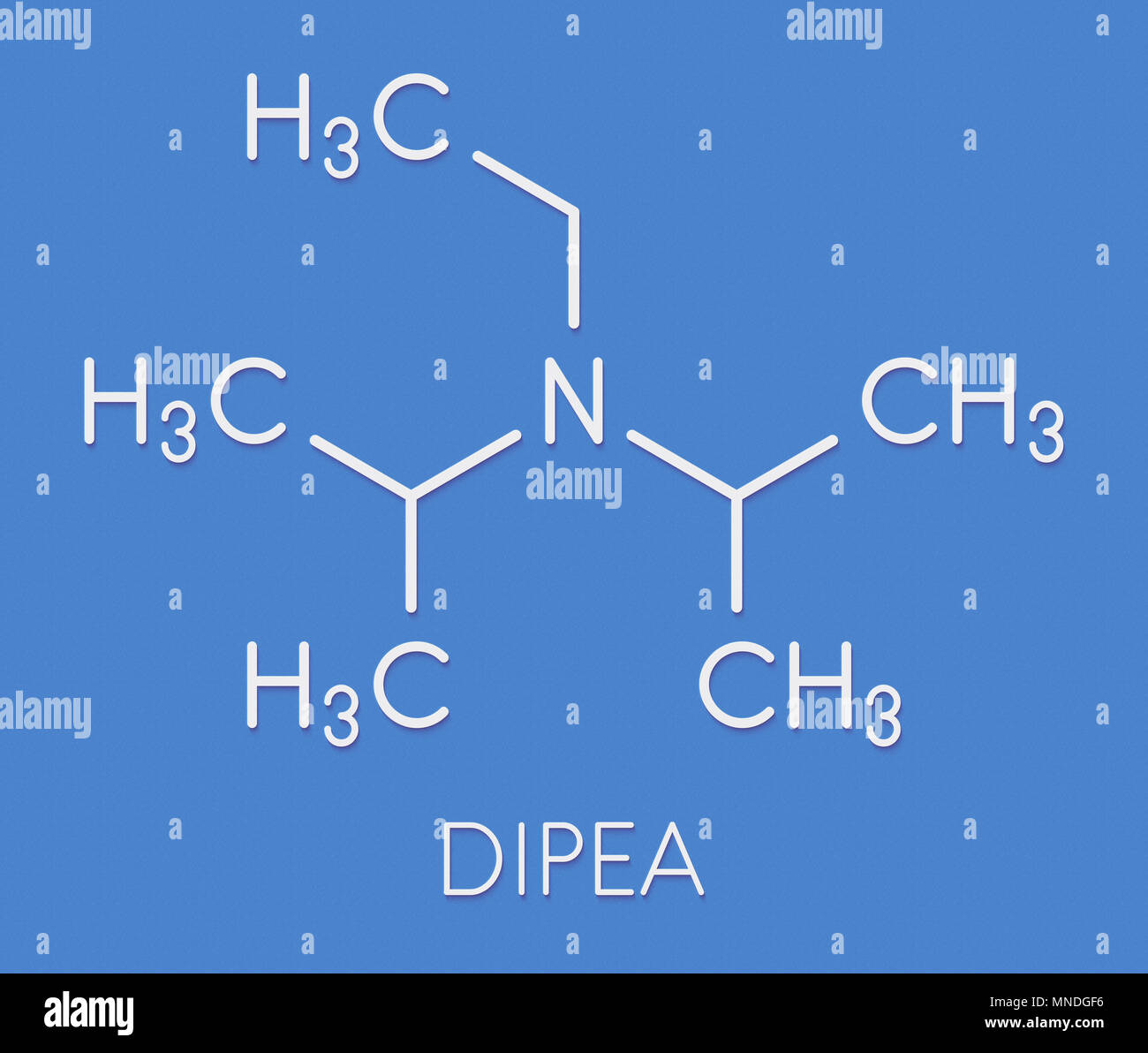
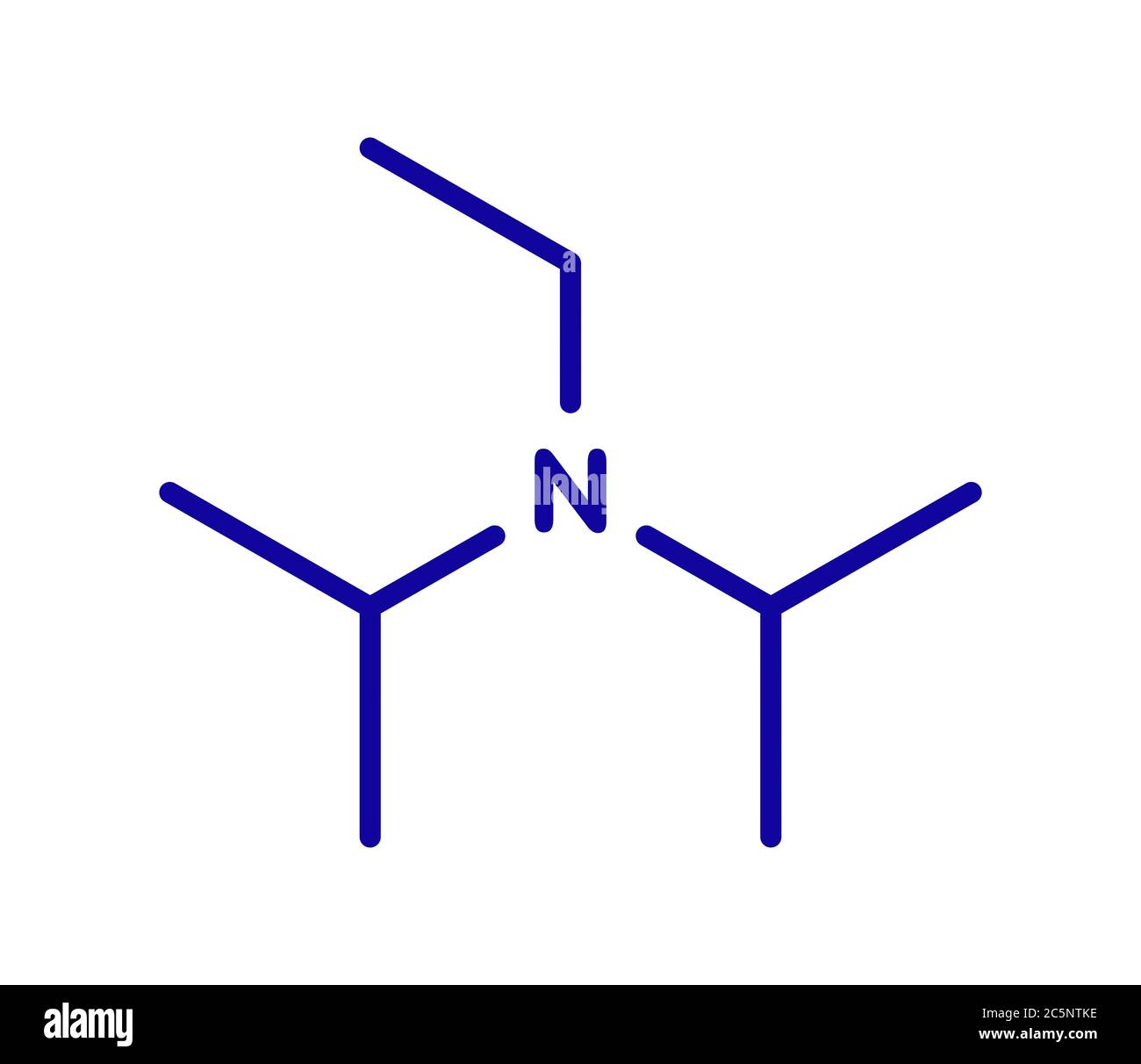
DIPEA (N,N-diisopropylethylamine, Hunig's Base) Molecule. Skeletal Formula. Royalty Free SVG, Cliparts, Vectors, and Stock Illustration. Image 149287710.
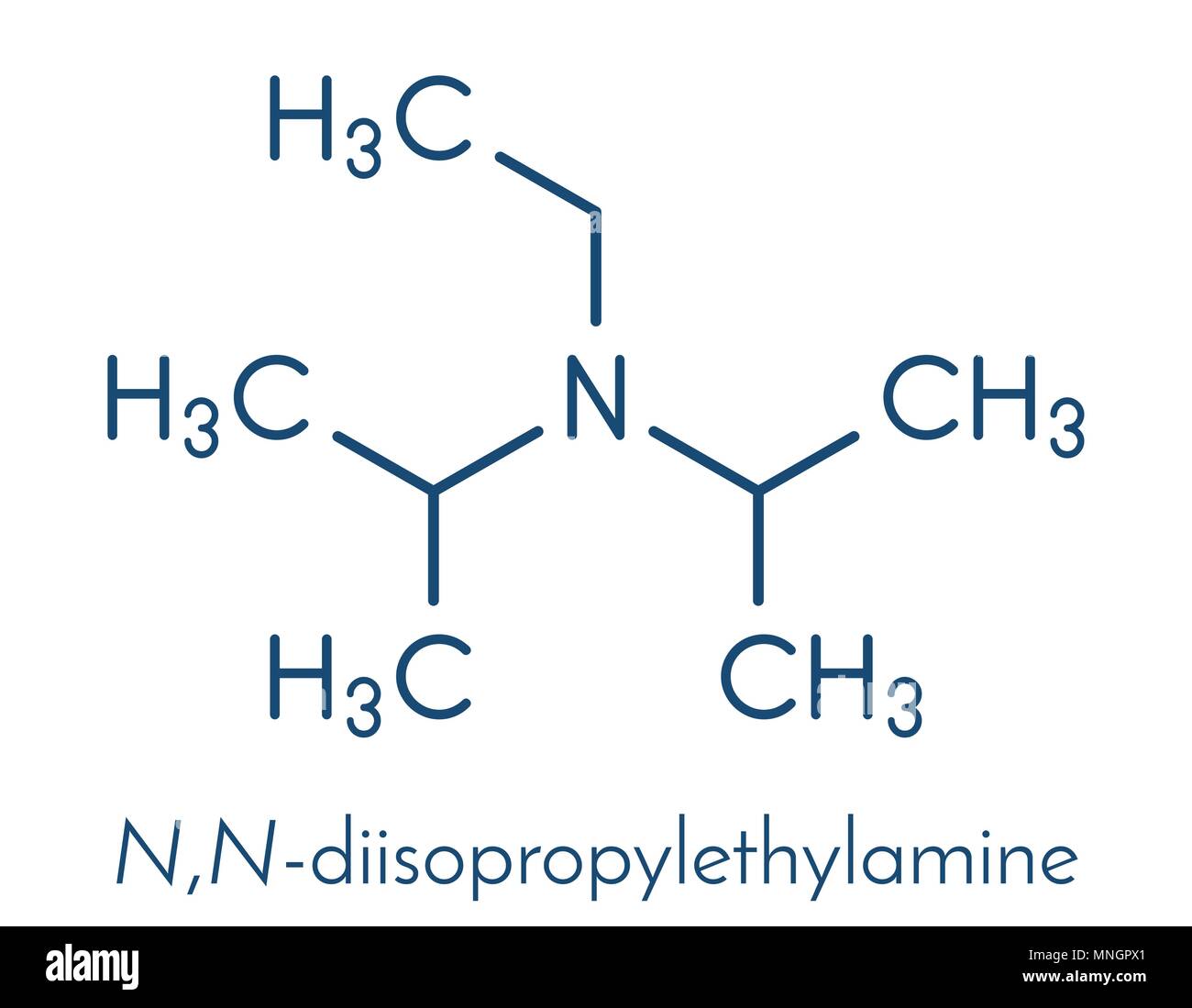
DIPEA (N,N-diisopropylethylamine, Hünig's base) molecule. Skeletal formula Stock Vector Image & Art - Alamy
Supporting Information Continuous-flow synthesis of primary amines: Metal-free reduction of aliphatic and aromatic nitro derivat
![PDF] A SIMPLE AND HIGHLY EFFICIENT SYNTHESIS OF QUINOLINE TERTIARY AMINES CATALYZED BY HUNIG'S BASE | Semantic Scholar PDF] A SIMPLE AND HIGHLY EFFICIENT SYNTHESIS OF QUINOLINE TERTIARY AMINES CATALYZED BY HUNIG'S BASE | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/3c974f939a32262aeed197ab08f1ab631c012982/3-Table1-1.png)
PDF] A SIMPLE AND HIGHLY EFFICIENT SYNTHESIS OF QUINOLINE TERTIARY AMINES CATALYZED BY HUNIG'S BASE | Semantic Scholar
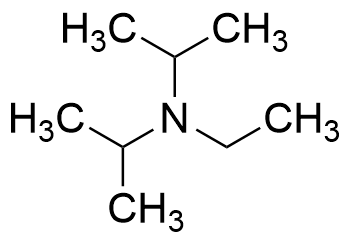
DIPEA (N,N-diisopropylethylamine, Hunig's base) molecule. Stylized skeletal formula (chemical structure): Atoms are shown as color-coded circles: hydrogen (hidden), carbon (grey Stock Photo - Alamy