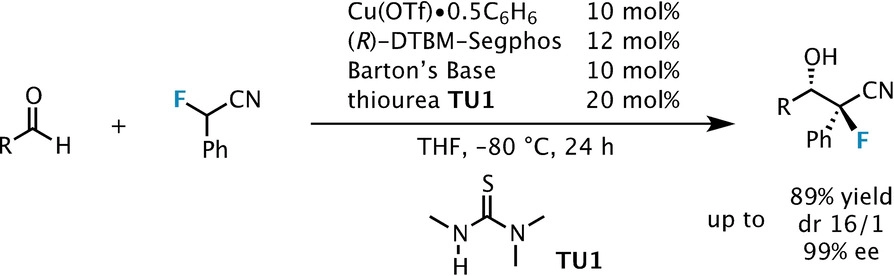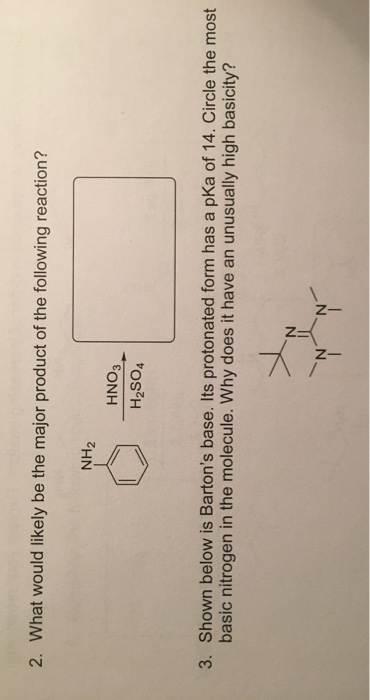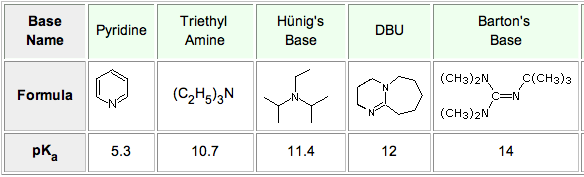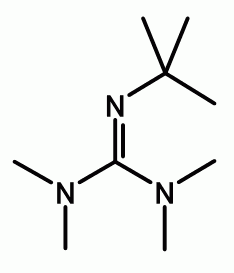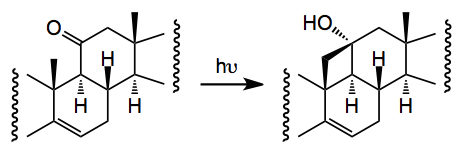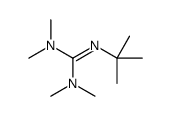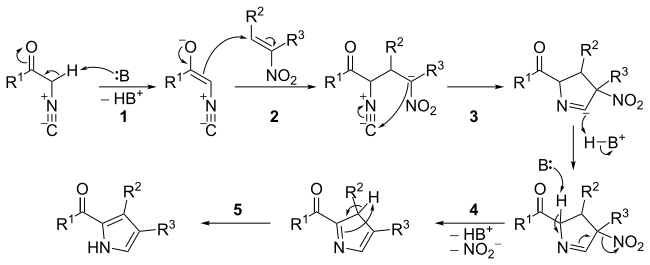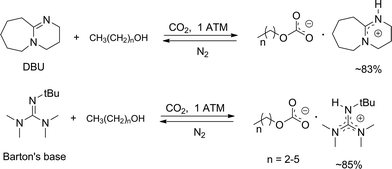
Anhydrous tertiary alkanolamines as hybrid chemical and physical CO 2 capture reagents with pressure-swing regeneration - Energy & Environmental Science (RSC Publishing) DOI:10.1039/C0EE00506A

Proposed catalytic cycle and TS of the present vinylogous addition of... | Download Scientific Diagram
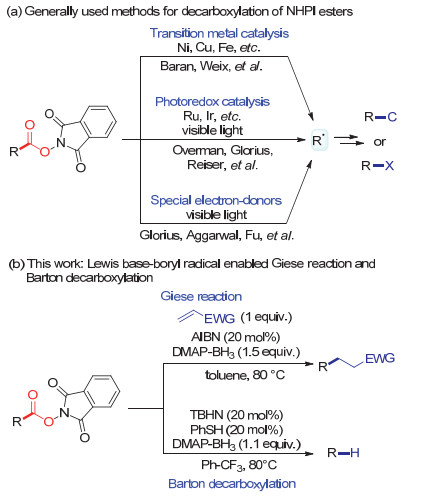
Lewis Base-Boryl Radical Enabled Giese Reaction and Barton Decarboxylation of N-Hydroxyphthalimide (NHPI) Esters

The 140th Annual Meeting of the Pharmaceutical Society of Japan (Kyoto)/Catalytic Asymmetric Synthesis of Chromanone Lactones Using Environmentally Friendly Vinylogous Michael Reactions
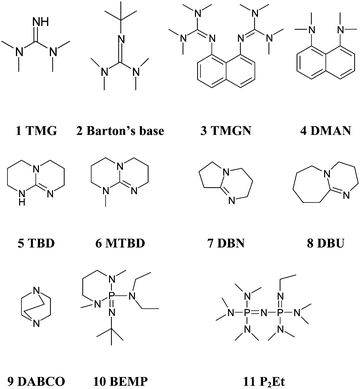
Kinetics screening of the N -alkylation of organic superbases using a continuous flow microfluidic device: basicity versus nucleophilicity - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C2OB25215E

Synthesis of Highly Oxygenated Dinaphthyl Ethers via SNAr Reactions Promoted by Barton's Base | Organic Letters

Synthesis of Highly Oxygenated Dinaphthyl Ethers via SNAr Reactions Promoted by Barton's Base | Organic Letters
![Bases investigated in this study. I, diazabicyclo[5.4.0]-undec7-ene... | Download Scientific Diagram Bases investigated in this study. I, diazabicyclo[5.4.0]-undec7-ene... | Download Scientific Diagram](https://www.researchgate.net/publication/228508616/figure/fig4/AS:668842074333185@1536475785337/Bases-investigated-in-this-study-I-diazabicyclo540-undec7-ene-DBU-II-1-1-3-3.png)