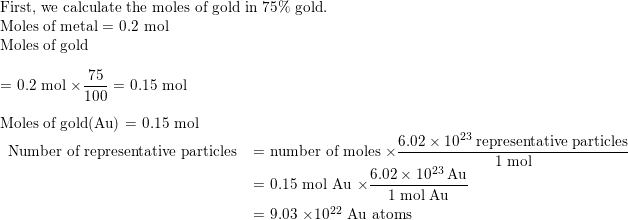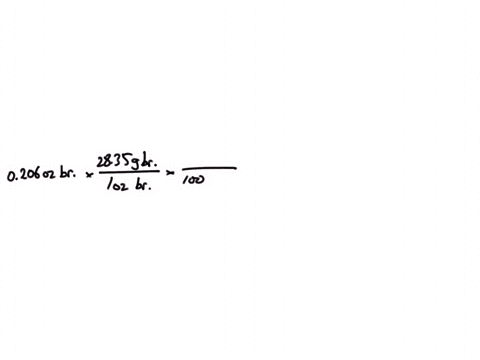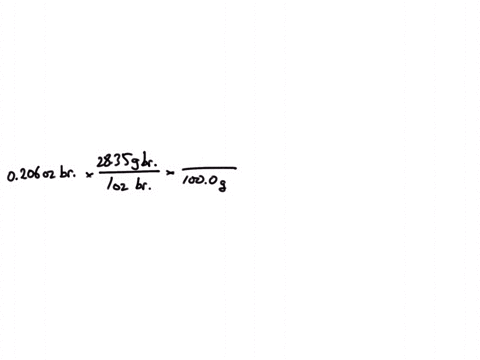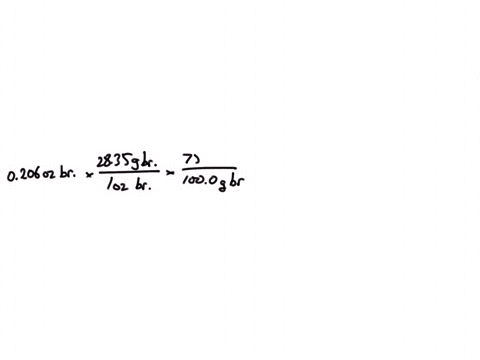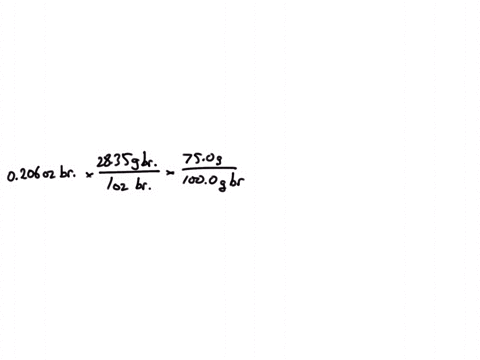
SOLVED:Jewelry A bracelet containing 0.200 mol metal atoms is 75% gold. How many particles of gold atoms are in the bracelet?

Excited State Aromaticity and Antiaromaticity: Opportunities for Photophysical and Photochemical Rationalizations | Chemical Reviews
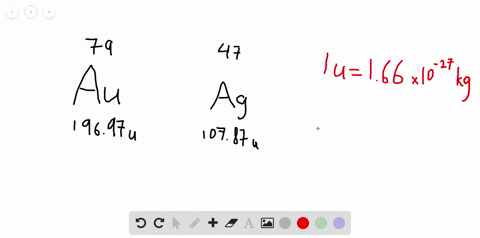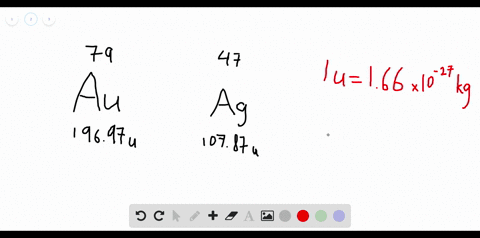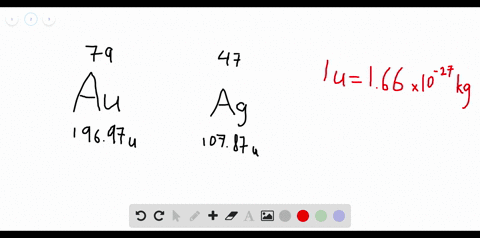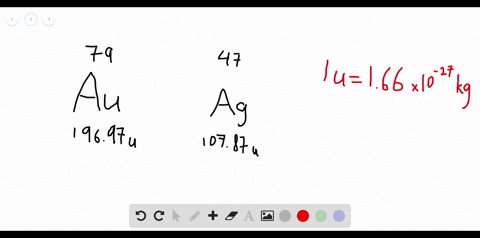
SOLVED: Jewelry A bracelet containing 0.200 mol metal atoms is 75% gold. How many particles of gold atoms are in the bracelet?
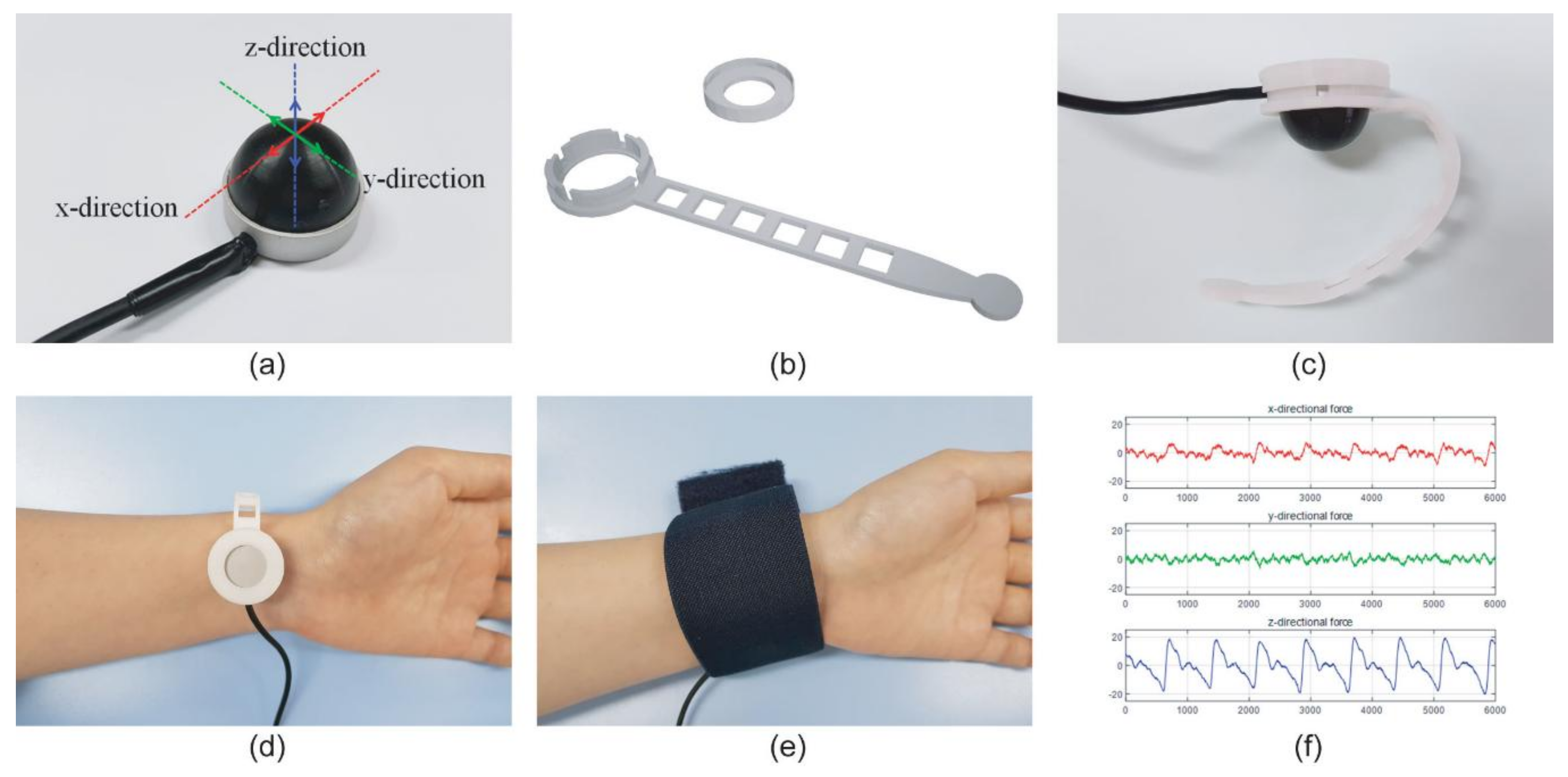
Sensors | Free Full-Text | Reliability and Validity of Non-invasive Blood Pressure Measurement System Using Three-Axis Tactile Force Sensor

SOLVED:Jewelry A bracelet containing 0.200 mol metal atoms is 75% gold. How many particles of gold atoms are in the bracelet?
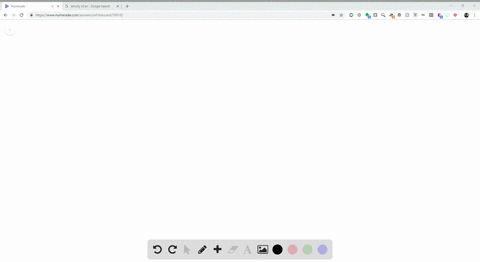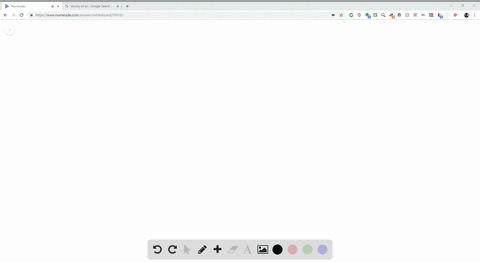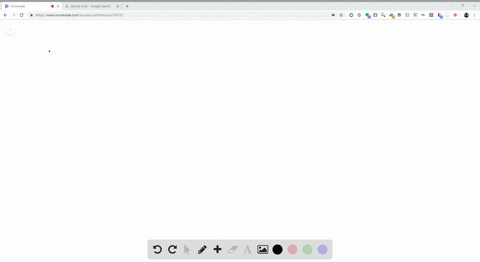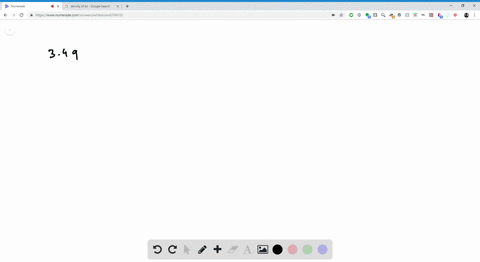
SOLVED: Jewelry A bracelet containing 0.200 mol metal atoms is 75% gold. How many particles of gold atoms are in the bracelet?
![Optically active electrochromic polymer films from cyclopenta[2,1-b;3,4-b′]ditiophene prepared in chiral liquid crystal with molecular asymmetry imprinting polymerization - ScienceDirect Optically active electrochromic polymer films from cyclopenta[2,1-b;3,4-b′]ditiophene prepared in chiral liquid crystal with molecular asymmetry imprinting polymerization - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0013468623005856-ga1.jpg)
Optically active electrochromic polymer films from cyclopenta[2,1-b;3,4-b′]ditiophene prepared in chiral liquid crystal with molecular asymmetry imprinting polymerization - ScienceDirect

PDF) The MoleThe Mole - Weeblyschoolisinsession.weebly.com/uploads/1/2/3/5/12350465/chapter_10... · The MoleThe Mole Solutions Manual Chemistry: Matter and Change • Chapter 10 161 Section - DOKUMEN.TIPS
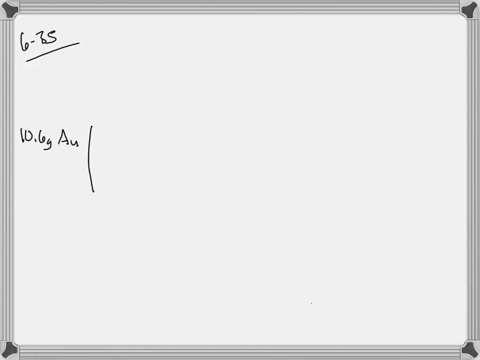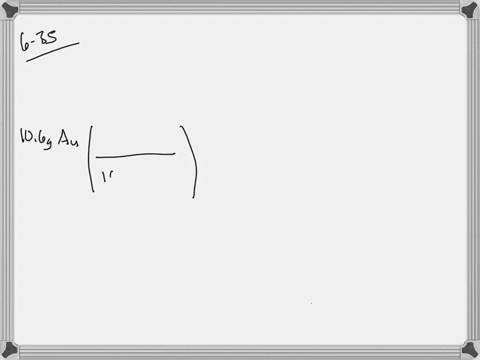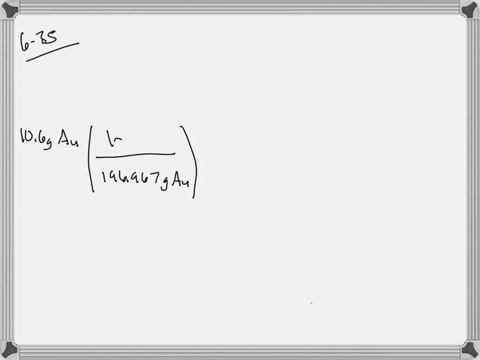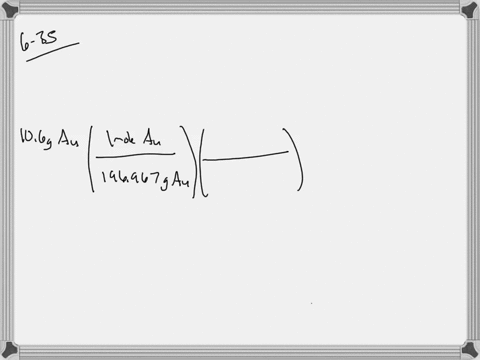
SOLVED: Jewelry A bracelet containing 0.200 mol metal atoms is 75% gold. How many particles of gold atoms are in the bracelet?